_noble electronic
1.英文电子书(畅销书籍的数字化时代)
2.专营店的简介
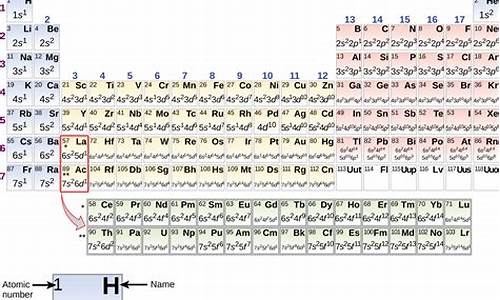
The periodic law is most commonly expressed in chemistry in the form of a periodic table, or chart. The so-called short-form periodic table, based on Mendeleyev's table, with subsequent emendations and additions, is still in widespread use. In this table the elements are arranged in seven horizontal rows, called the periods, in order of increasing atomic weights, and in 18 vertical columns, called the groups. The first period, containing two elements, hydrogen and helium, and the next two periods, each containing eight elements, are called the short periods. The remaining periods, called the long periods, contain 18 elements, as in periods 4 and 5, or 32 elements, as in period 6. The long period 7 includes the actinide series, which has been filled in by the synthesis of radioactive nuclei through element 102, nobelium. Heier transuranium elements he also been synthesized.
The groups or vertical columns of the periodic table he traditionally been labeled from left to right using Roman numerals followed by the symbol a or b, the b referring to groups of transition elements. Another labeling scheme, which has been adopted by the International Union of Pure and Applied Chemistry (IUPAC), is gaining in popularity. This new system simply numbers the groups sequentially from 1 to 18 across the periodic table.
All the elements within a single group bear a considerable familial resemblance to one another and, in general, differ markedly from elements in other groups. For example, the elements of group 1 (or Ia), with the exception of hydrogen, are metals with chemical valence of +1; while those of group 17 (or VIIa), with the exception of astatine, are nonmetals, commonly forming compounds in which they he valences of -1.
In the periodic classification, noble gases, which in most cases are unreactive (valence = 0), are interposed between highly reactive metals that form compounds in which their valence is +1 on one side and highly reactive nonmetals forming compounds in which their valence is -1 on the other side. This phenomenon led to the theory that the periodicity of properties results from the arrangement of electrons in shells about the atomic nucleus. According to the same theory, the noble gases are normally inert because their electron shells are completely filled; other elements, therefore, may he some shells that are only partly filled, and their chemical reactivities involve the electrons in these incomplete shells. Thus, all the elements that occupy a position in the table preceding that of an inert gas he one electron less than the number necessary for completed shells and show a valence of -1, corresponding to the gain of one electron in reactions. Elements in the group following the inert gases in the table he one electron in excess of the completed shell structure and in reactions can lose that electron, thereby showing a valence of + 1.
An analysis of the periodic table, based on this theory, indicates that the first electron shell may contain a maximum of 2 electrons, the second builds up to a maximum of 8, the third to 18, and so on. The total number of elements in any one period corresponds to the number of electrons required to achieve a stable configuration. The distinction between the a and b subgroups of a given group also may be explained on the basis of the electron shell theory. Both subgroups he the same degree of incompleteness in the outermost shell but differ from each other with respect to the structures of the underlying shells. This model of the atom still provides a good explanation of chemical bonding.
There are 18 vertical columns, or groups, in the standard periodic table. At present, there are three versions of the periodic table, each with its own unique column headings, in wide use. The three formats are the old International Union of Pure and Applied Chemistry (IUPAC) table, the Chemical Abstract Service (CAS) table, and the new IUPAC table. The old IUPAC system labeled columns with Roman numerals followed by either the letter A or B. Columns 1 through 7 were numbered IA through VIIA, columns 8 through 10 were labeled VIIIA, columns 11 through 17 were numbered IB through VIIB and column 18 was numbered VIII. The CAS system also used Roman numerals followed by an A or B. This method, however, labeled columns 1 and 2 as IA and IIA, columns 3 through 7 as IIIB through VIB, column 8 through 10 as VIII, columns 11 and 12 as IB and IIB and columns 13 through 18 as IIIA through VIIIA. However, in the old IUPAC system the letters A and B were designated to the left and right part of the table, while in the CAS system the letters A and B were designated to the main group elements and transition elements respectively. (The preparer of the table arbitrarily could use either an upper-or lower-case letter A or B, adding to the confusion.) Further, the old IUPAC system was more frequently used in Europe while the CAS system was most common in America. In the new IUPAC system, columns are numbered with Arabic numerals from 1 to 18. These group numbers correspond to the number of s, p, and d orbital electrons added since the last noble gas element (in column 18). This is in keeping with current interpretations of the periodic law which holds that the elements in a group he similar configurations of the outermost electron shells of their atoms. Since most chemical properties result from outer electron interactions, this tends to explain why elements in the same group exhibit similar physical and chemical properties. Unfortunately, the system fails for the elements in the first 3 periods (or rows; see below). For example, aluminum, in the column numbered 13, has only 3 s, p, and d orbital electrons. Nevertheless, the American Chemical Society has adopted the new IUPAC system.
The horizontal rows of the table are called periods. The elements of a period are characterized by the fact that they he the same number of electron shells; the number of electrons in these shells, which equals the element's atomic number, increases from left to right within each period. In each period the lighter metals ear on the left, the heier metals in the center, and the nonmetals on the right. Elements on the borderline between metals and nonmetals are called metalloids.
Group 1 (with one valence electron) and Group 2 (with two valence electrons) are called the alkali metals and the alkaline-earth metals, respectively. Two series of elements branch off from Group 3, which contains the transition elements, or transition metals; elements 57 to 71 are called the lanthanide series, or rare earths, and elements 89 to 103 are called the actinide series, or radioactive rare earths; a third set, the superactinide series (elements 122–153), is predicted to fall outside the main body of the table, but none of these has yet been synthesized or isolated. The nonmetals in Group 17 (with seven valence electrons) are called the halogens. The elements grouped in the final column (Group 18) he no valence electrons and are called the inert gases, or noble gases, because they react chemically only with extreme difficulty.
In a relatively simple type of periodic table, each position gives the name and chemical symbol for the element assigned to that position; its atomic number; its atomic weight (the weighted erage of the masses of its stable isotopes, based on a scale in which carbon-12 has a mass of 12); and its electron configuration, i.e., the distribution of its electrons by shells. The only exceptions are the positions of elements 103 through 118; complete information on these elements has not been compiled. Larger and more complicated periodic tables may also include the following information for each element: atomic diameter or radius; common valence numbers or oxidation states; melting point; boiling point; density; specific heat; Young's modulus; the quantum states of its valence electrons; type of crystal form; stable and radioactive isotopes; and type of magnetism exhibited by the element (paramagnetism or diamagnetism).
The layout of the periodic table demonstrates recurring ("periodic") chemical properties. Elements are listed in order of increasing atomic number (i.e., the number of protons in the atomic nucleus). Rows are arranged so that elements with similar properties fall into the same columns (groups or families). According to quantum mechanical theories of electron configuration within atoms, each row (period) in the table corresponded to the filling of a quantum shell of electrons. There are progressively longer periods further down the table, grouping the elements into s-, p-, d- and f-blocks to reflect their electron configuration.
In printed tables, each element is usually listed with its element symbol and atomic number; many versions of the table also list the element's atomic mass and other information, such as its abbreviated electron configuration, electronegativity and most common valence numbers.
As of 2006, the table contains 117 chemical elements whose discoveries he been confirmed. Ninety-four are found naturally on Earth, and the rest are synthetic elements that he been produced artificially in particle accelerators. Elements 43 (technetium), 61 (promethium) and all elements greater than 83 (bismuth), beginning with 84 (polonium) he no stable isotopes. The atomic mass of each of these element's isotope hing the longest half-life is typically reported on periodic tables with parentheses.[1] Isotopes of elements 43, 61, 93 (neptunium) and 94 (plutonium), first discovered synthetically, he since been discovered in trace amounts on Earth as products of natural radioactive decay processes.
The primary determinant of an element's chemical properties is its electron configuration, particularly the valence shell electrons. For instance, any atoms with four valence electrons occupying p orbitals will exhibit some similarity. The type of orbital in which the atom's outermost electrons reside determines the "block" to which it belongs. The number of valence shell electrons determines the family, or group, to which the element belongs.
The total number of electron shells an atom has determines the period to which it belongs. Each shell is divided into different subshells, which as atomic number increases are filled in roughly this order (the Aufbau principle):
Groups
Main article: Group (periodic table)
A group or family is a vertical column in the periodic table. Groups are considered the most important method of classifying the elements. In some groups, the elements he very similar properties and exhibit a clear trend in properties down the group. These groups tend to be given trivial (unsystematic) names, e.g., the alkali metals, alkaline earth metals, halogens, pnictogens, chalcogens, and noble gases. Some other groups in the periodic table display fewer similarities and/or vertical trends (for example Group 14), and these he no trivial names and are referred to simply by their group numbers.
Periods
Main article: Period (periodic table)
A period is a horizontal row in the periodic table. Although groups are the most common way of classifying elements, there are some regions of the periodic table where the horizontal trends and similarities in properties are more significant than vertical group trends. This can be true in the d-block (or "transition metals"), and especially for the f-block, where the lanthanoids and actinoids form two substantial horizontal series of elements.
Blocks
Main article: Periodic table block
This diagram shows the periodic table blocks.Because of the importance of the outermost shell, the different regions of the periodic table are sometimes referred to as periodic table blocks, named according to the subshell in which the "last" electron resides. The s-block comprises the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p-block comprises the last six groups (groups 13 through 18) and contains, among others, all of the semimetals. The d-block comprises groups 3 through 12 and contains all of the transition metals. The f-block, usually offset below the rest of the periodic table, comprises the rare earth metals.
Other
The chemical elements are also grouped together in other ways. Some of these groupings are often illustrated on the periodic table, such as transition metals, poor metals, and metalloids. Other informal groupings exist, such as the platinum group and the noble metals.
Periodicity of chemical properties
The main value of the periodic table is the ability to predict the chemical properties of an element based on its location on the table. It should be noted that the properties vary differently when moving vertically along the columns of the table than when moving horizontally along the rows.
Periodic trends of groups
Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group he the same electron configurations in their valence shell, which is the most important factor in accounting for their similar properties. Elements in the same group also show patterns in their atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, valence electrons are found farther from the nucleus. From the top, each successive element has a lower ionization energy because it is easier to remove an electron since the atoms are less tightly bound. Similarly, a group will also see a top to bottom decrease in electronegativity due to an increasing distance between valence electrons and the nucleus.
Periodic trends of periods
Periodic trend for ionization energy. Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases.Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus. This decrease in atomic radius also causes the ionization energy to increase when moving from left to right across a period. The more tightly bound an element is, the more energy is required to remove an electron. Similarly, electronegativity will increase in the same manner as ionization energy because of the amount of pull that is exerted on the electrons by the nucleus. Electron affinity also shows a slight trend across a period. Metals (left side of a period) generally he a lower electron affinity than nonmetals (right side of a period) with the exception of the noble gases.
英文电子书(畅销书籍的数字化时代)
2017高中留学必备化学英语词汇
化学是自然科学的一种,是高中必修的科目之一。下面是我整理的高中化学英语词汇,希望能帮到想要出国留学的大家!
limestone:石灰石
linear:直线的
lipid:脂质,类脂
lithium:锂
litmus:石蕊
magnesium:镁
metalloid:类金属
methane:甲烷
methanoic acid:甲酸
methanol:甲醇
monomer:单体
neon:氖
neutralization:中和
nickel:镍
nitrate:硝酸盐
nitric acid:硝酸
nitrogen:氮
noble gases:稀有气体
oxide:氧化物
oxidation agent:氧化剂
ozone:臭氧
periodic table:元素周期表
permanganate:高锰酸
petroleum:石油
phenolphthalein:酚酞
phosphorus:磷
planar:平面的.
platinum:铂
polar:极性的
polymer:聚合物
potassium:钾
precipitation:沉淀
propane:丙烷
propanoic acid:丙酸
propanol:丙醇
propene:丙烯
pure substance:纯净物
reactive:活泼的
redox:氧化还原
reducing agent:还原剂
reversible reaction:可逆反应
shared paris of electrons:共用电子对
silicon:硅
sodium:钠
solvent:溶剂
starch:淀粉
sublime:升华
sulphate:硫酸盐
sulphur:硫
sulphuric acid:硫酸
suspension:悬浮液
tetrahedral:四面体
titration:滴定
torr:托耳(压强单位)
triple bonds:三键
van der Waals' force:范德华力
volatile:易挥发的
zinc:锌
acid:酸
activated complex:活化络合物
activation energy:活化能
aggregate:聚合
alkali metals:碱金属
alkaline earth metals:碱土金属
alkane:烷烃
alkene:烯烃
alkyne:炔烃
allotropy:同素异形体
alloy:合金
aluminum:铝
ammonia:氨
amorphous:非晶体
amphoteric:的
anode:阳极
anomalous:不规则的
aqueous:水溶液
argon:氩
Avogadro's law:阿佛加德罗定律
barium:钡
bauxite:铝土矿
bleaching:漂白
bond:键
boron:硼
brass:黄铜
bromine:湨
buret:滴定管
calcium:钙
calory:卡路里
carbohydrate:碳水化合物
carbon dioxide:二氧化碳
carbon monoxide:一氧化碳
carbonate:碳酸盐
carboxylic acid:羧酸
catalyst:催化剂
cathode reaction:阴极反应
cellulose:纤维素
chlorine:氯
chromium:铬
coefficient:系数
combination reaction:化合反应
combustion:燃烧
composition:组成
compound:化合物
concentrated:浓的
concentration:浓度
condensation:冷凝
covalent bond:共价键
cracking:裂解
crude oil:原油
crystal:晶体
deoxyribose:脱氧核糖
diatomic molecule:双原子分子
diffusion:扩散
dilute:稀的
displacement reaction:置换反应
distillation:蒸馏
double bonds:双键
dynamic equilibrium:动态平衡
electrolysis:电解
elcetrolyte:电解质
electronic configuration:电子排布
element:元素
empirical formula:经验式
endothermic:吸热的
enthalpy:焓
erosion:腐蚀
ethanoic acid:乙酸
ethanol:乙醇
ethene:乙烯
evaporation:蒸发
exothermic:放热的
extraction:提炼
filtering:过滤
flame test:焰色反应
fluorine:氟
fractional distillation:分馏
freezing point:凝固点
glucose:葡萄糖
glycogen:糖原
graphite:石墨
greenhouse effect:温室效应
Haber process:哈伯法(制氨)
halogen:卤素
helium:氦
homologous series:同系物
hydrocarbon:烃
hydrogen:氢
hydrogen chloride:氯化氢
hydrogen peroxide:过氧化氢
hydronium ion:水合氢离子
hydroxide:氢氧根
indicator:指示剂
iodine:碘
isomerism:同素异形体
le Chatelier's principle:勒沙特列原理
lead:铅
;专营店的简介
TheDigitalAgeofBestsellingBooks:EmbracingtheEraofE-books
Introduction
Intoday'sdigitalera,theriseofelectronicbooks,ore-books,hasrevolutionizedthewaywereadandaccessinformation.Withtheadventofsmartphones,tablets,ande-readers,readerscannowcarryanentirelibraryintheirpocket.Inthisarticle,wewillexploretheworldofe-books,theiradvantages,andhowtoaccessandenjoythem.
WhatareE-books?
E-books,shortforelectronicbooks,aredigitalversionsofprintedbooksthatcanbereadonelectronicdevices.TheycomeinvariousformatssuchasPDF,EPUB,andMOBI,makingthemcompatiblewithdifferente-readersanddevices.E-booksofferarangeoffeaturesthatenhancethereadingexperience,includingadjustablefontsizes,bookmarking,highlighting,andsearchingforspecificwordsorphrases.
AdvantagesofE-books
1.Portability:Oneofthebiggestadvantagesofe-booksistheirportability.Withasingledevice,readerscancarrythousandsofbookswherevertheygo.Thiseliminatestheneedforheyphysicalbooksandallowsreaderstoreadonthego.
2.Accessibility:E-bookshemadereadingmoreaccessibletopeoplewithvisualimpairmentsorreadingdifficulties.Manye-readershebuilt-inaccessibilityfeaturessuchastext-to-speech,adjustablefonts,andbackgroundcolorcustomization.
3.Cost-effective:E-booksareoftencheaperthantheirprintedcounterparts.Withe-books,publishersseonprintinganddistributioncosts,allowingthemtoofferbooksatlowerprices.Additionally,readerscanfindmanyfreeordiscountede-booksonline.
4.Environmentallyfriendly:Bychoosinge-booksoverprintedbooks,readerscontributetoenvironmentalconservation.E-bookssetrees,reducecarbonemissionsfromtransportation,andminimizewastefrombookproduction.
AccessingE-books
1.E-bookRetailers:Manyonlineretailersspecializeinsellinge-books.Amazon'sKindleStore,Apple'siBooks,andBarnes&Noble'sNookStorearepopularplatformswherereaderscanpurchaseanddownloade-booksdirectlytotheirdevices.
2.PublicLibraries:Mostpubliclibrariesnowoffere-booksforborrowing.ThroughplatformslikeOverDriveorLibby,librarypatronscanborrowe-booksandreadthemontheirdevicesforalimitedperiod.
3.E-bookSubscriptionServices:SubscriptionserviceslikeKindleUnlimitedandScribdofferunlimitedaccesstoastlibraryofe-booksforamonthlyfee.Theseservicesareperfectforidreaderswhowanttoexploreawiderangeoftitleswithoutpurchasingeachbookindividually.
EnjoyingE-books
1.ChoosingtheRightDevice:Toenjoye-books,you'llneedacompatibledevice.Populare-readersincludeAmazonKindle,Kobo,andBarnes&NobleNook.Alternatively,youcanreade-booksonyoursmartphoneortabletusingdedicatede-readerslikeKindle,AppleBooks,orGooglePlayBooks.
2.CustomizingtheReadingExperience:Moste-readersallowuserstocustomizetheirreadingexperience.Youcanadjustfontsize,fonttype,andlinespacingtosuityourpreferences.Somee-readersalsoofferfeatureslikenightmode,whichreduceseyestraininlow-lightconditions.
3.TakingAdvantageofFeatures:E-booksofferfeaturesthatenhancethereadingexperience.Youcanbookmarkpages,highlightpassages,andmakenotes,justlikeyouwouldwithaphysicalbook.Additionally,e-readersoftenhebuilt-indictionariesandtranslationtoolsforquickwordlookupsandlanguageassistance.
Conclusion
Inthedigitalage,e-bookshebecomeapopularchoiceforreadersworldwide.Theirportability,accessibility,andcost-effectivenessmakethemanattractivealternativetotraditionalprintedbooks.Byembracinge-books,readerscanenjoyastlibraryattheirfingertipsandcontributetoamoresustainablefuture.So,whetheryou'reabookloveroracasualreader,it'stimetodiveintotheworldofe-booksandexperiencethejoyofreadinginthedigitalage.
专营店(specialty store)
专营店定义:指占地800平方英尺以下,主要集中经营有限量的几种互补商品并提供高水准的服务的零售商。
与百货商店和折扣商店相对照,专营店集中于一块狭长的市场分区,经营少量的商品品种但商品种类多样。就商品种类而言,专营店比百货商店或折扣商店能为顾客提供更好的选择和销售专家评价。顾客则被专营店多样的商品种类、个性化和熟悉的商店氛围所吸引。
最大的专营连锁公司有The Gap公司和The Limited公司(专营服装),巴诺(Barnes & Noble)公司(专营书籍),Pier l 公司(专营家具),Zale公司(专营珠宝),Payless(专营鞋)和 Electronic Boutique公司(专营计算机软件)等。 所谓专营店,一般是指专门经营某一类或者某一种品牌商品的商店,它实际上包括了国家行业标准中的专业店、专营店和家居建材商店。 国家标准对专业店的定义是:以专门经营某一大类商品为主的零售业态;
对专营店的定义是:以专门经营或被授权经某一主要品牌商品为主的零售业态,也叫做加盟店,合伙店,连锁店,形像店等;
对家居建材商店的定义是:以专门销售建材、装饰、家居用品为主的零售业态。
可见,这三类业态形势虽然有明显的区别,但从经营商品范围的专业性角度看他们共同点使其能够作为一个整体成为研究对象,都属于专业化和深度化经营的专营店。 从世界各国专营店发展的经验看,较为成熟的专营店具有以下特点:
经营商品“专” 由于“专”,使其经营的某类商品品种齐全,技术含量高,能够满足某一市场的特殊需求,专营店的这种优势是其他综合性商店不能比拟的,因此其目标顾客以有目的地选购某类商品的流动顾客为主。 服务方式“活” 专营店经营政策的核心是品种政策、差别化政策。购方式有各店购、集中购、集中与各店购结合等三种方式,比较重视各店的自主权与灵活性,能够提供针对性的服务,在专营店里,每个导购人员都是某类商品经营的行家,他们帮助顾客进行消费设计,根据顾客特点,为他们设计生活,指导消费。
规模较“小” 主要指单体规模小。在欧美国家虽然不乏万米以上的大型专营店,但大量的是规模较小且实行连锁经营的,包括廉价型、偏好型、家庭中心型等。专营店过大,商品就难以“专”,也就失去了特色。但家居建材商店因商品品种及经营方式等特点,其规模有扩大化趋势。
资本回收期“短” 拥有鲜明特色的专营店对于目标市场具有很强的吸引力,加之单店投资较小,能够在较短的时间内收回投资,不至于长期负债经营。专业化经营的方式又保证了其拥有忠诚度较高的消费群体,因此,专营店的经营风险也就不大。
正因为专营店具有以上特点和优势,因此,它在零售业中占有重要的地位,它既是百货商店、超市等综合性业态形式的补充,也是通过精细化经营,满足消费者更高层次需求的零售业态升级。
声明:本站所有文章资源内容,如无特殊说明或标注,均为采集网络资源。如若本站内容侵犯了原著者的合法权益,可联系本站删除。












